Overview
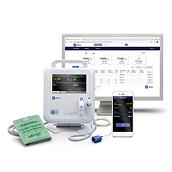
The Hillrom Extended Care Solution, part of the Hillrom™ Connected Care Platform, combines a clinical-grade, all-in-one vital signs device with a patient app and clinician review portal, so you can extend patient care beyond the walls of your facility.
Distance doesn’t mean disconnected.
- Seamlessly send vital signs measurements to the Hillrom Connex App with Bluetooth® (BLE)
- SureBP® technology can provide BP in only 15 seconds
- Easy-to-use Blood Pressure Averaging program
- SureTemp® Plus Oral/Axillary Thermometry
- Nonin® SpO2 technology measures blood oxygen saturation
- 7" color touchscreen for a simple, user-friendly workflow
- Each device ships with four FlexiPort® EcoCuff® single-patient blood pressure cuffs
- 8-hour Lithium ion battery to maximize time before recharging
- Patient-entered weight parameter
- iOS and Android compatibility
- Clinician notification email messages


SIMPLE
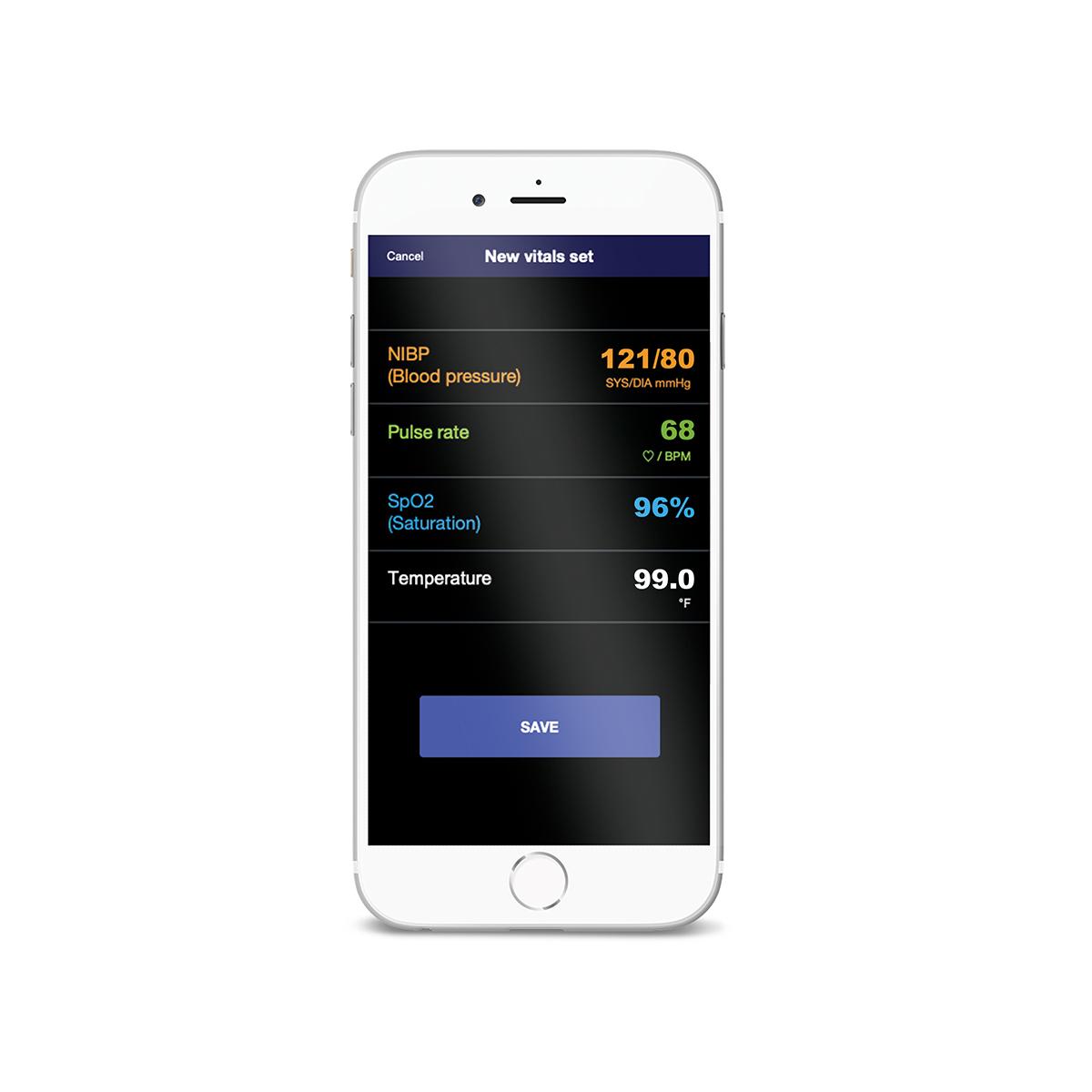
Patients' vitals are sent to the clinical portal with the push of a button.

SAFE
Help reduce hospital patient load, exposure and use of protective gear.

CONNECTED
Get the vitals data you need to help make informed care decisions.

SECURE
The secure, web-based portal provides streamlined access to patient data.
Hillrom™ Extended Care Solution
Introducing the Hillrom™ Extended Care Solution: Essential remote care at the most vital time. Technology and care tactics may evolve, but some things stay the same: Data-driven decision-making is at the heart of good healthcare.
Hillrom™ Extended Care Solution
Introducing the Hillrom™ Extended Care Solution: Essential remote care at the most vital time. Technology and care tactics may evolve, but some things stay the same: Data-driven decision-making is at the heart of good healthcare.
Funding for Fundamental Care: Reimbursements and the Hillrom™ Extended Care Solution
The way we provide healthcare is changing — and so are reimbursement codes. CPT codes are available for the care that you provide through the Hillrom™ Extended Care Solution. It’s essential to get remote healthcare right. See how we can help.
Monitor with Confidence
Get the vital data you need to help make informed care decisions — from a distance.
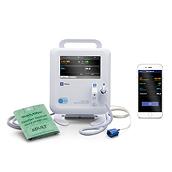
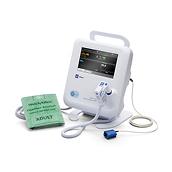
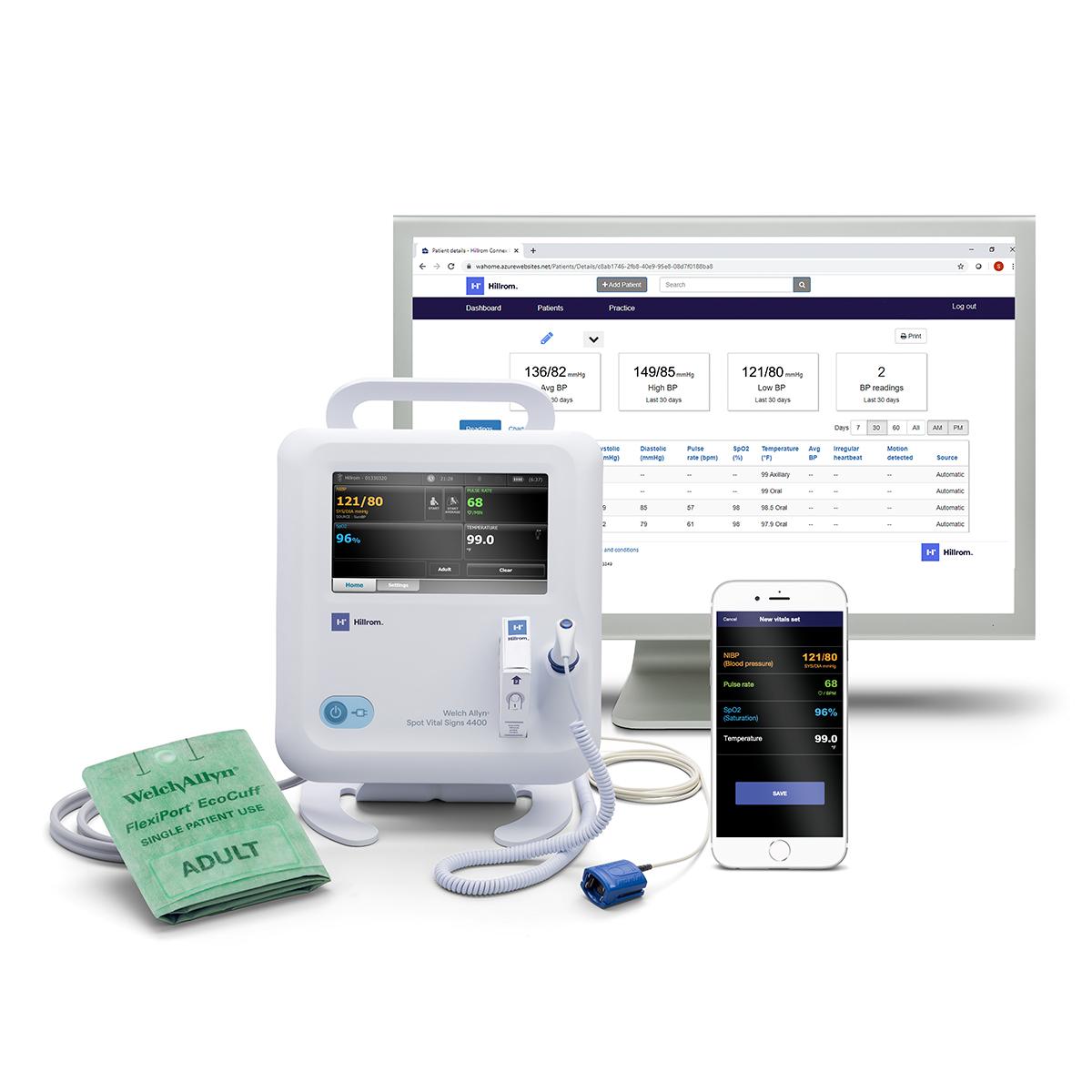
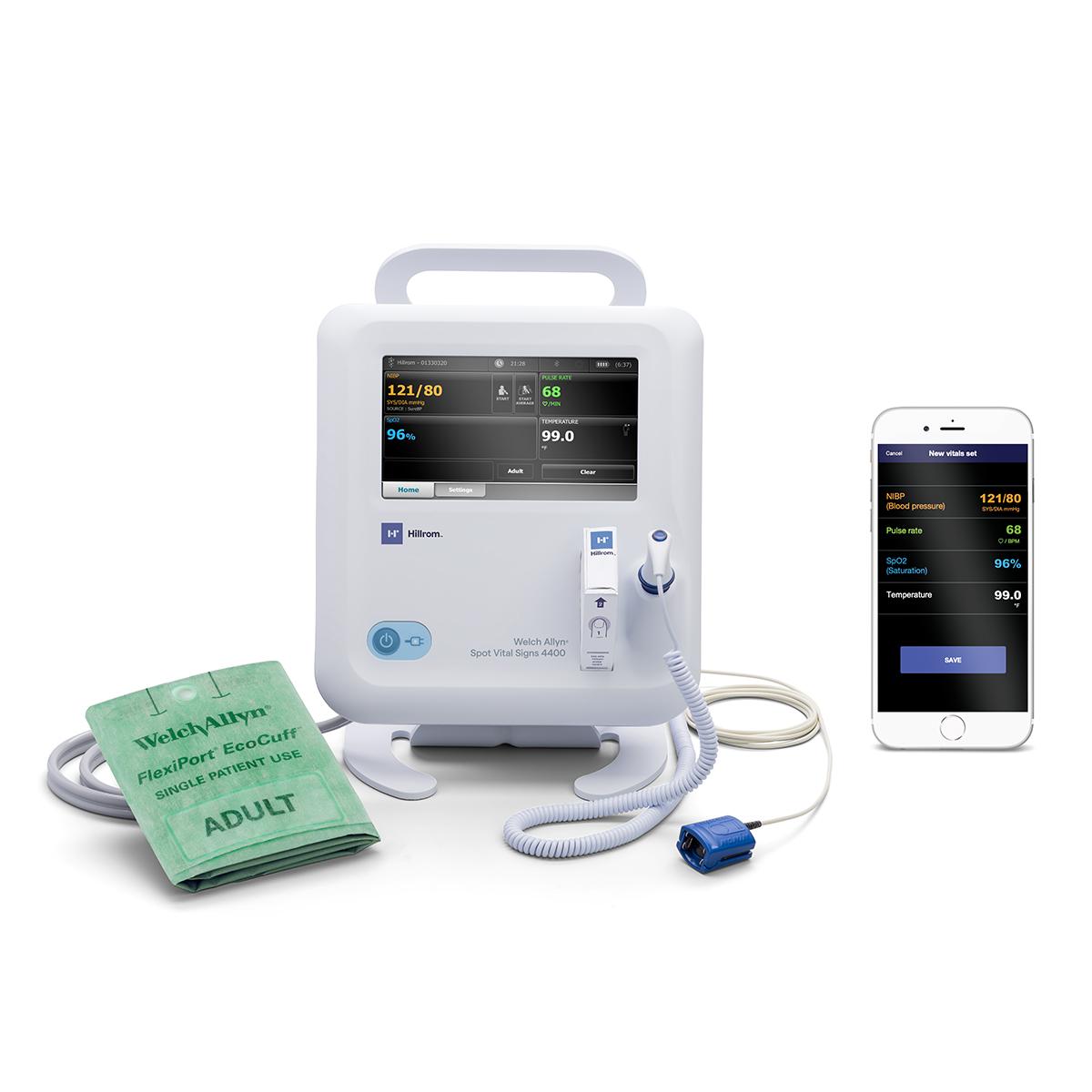
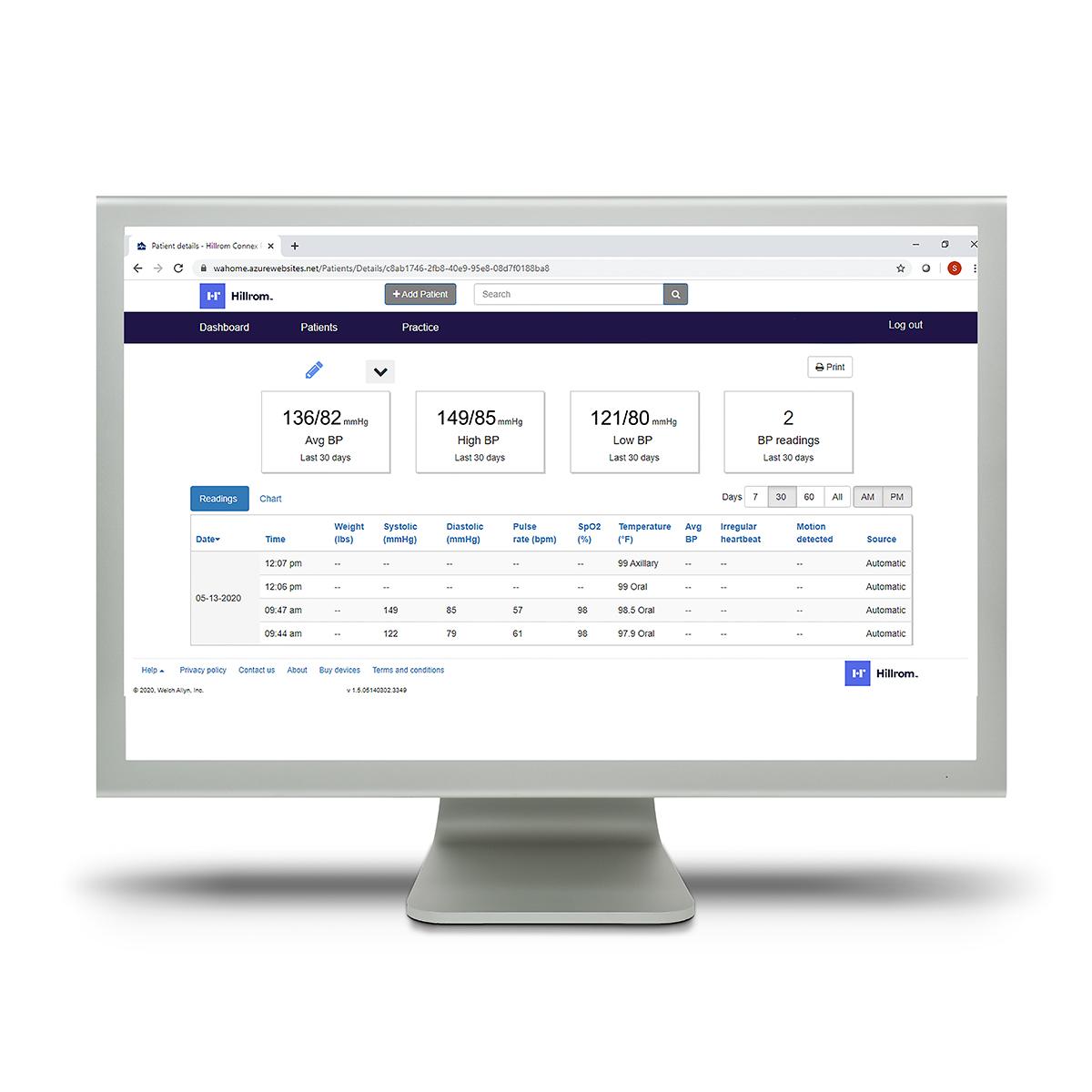
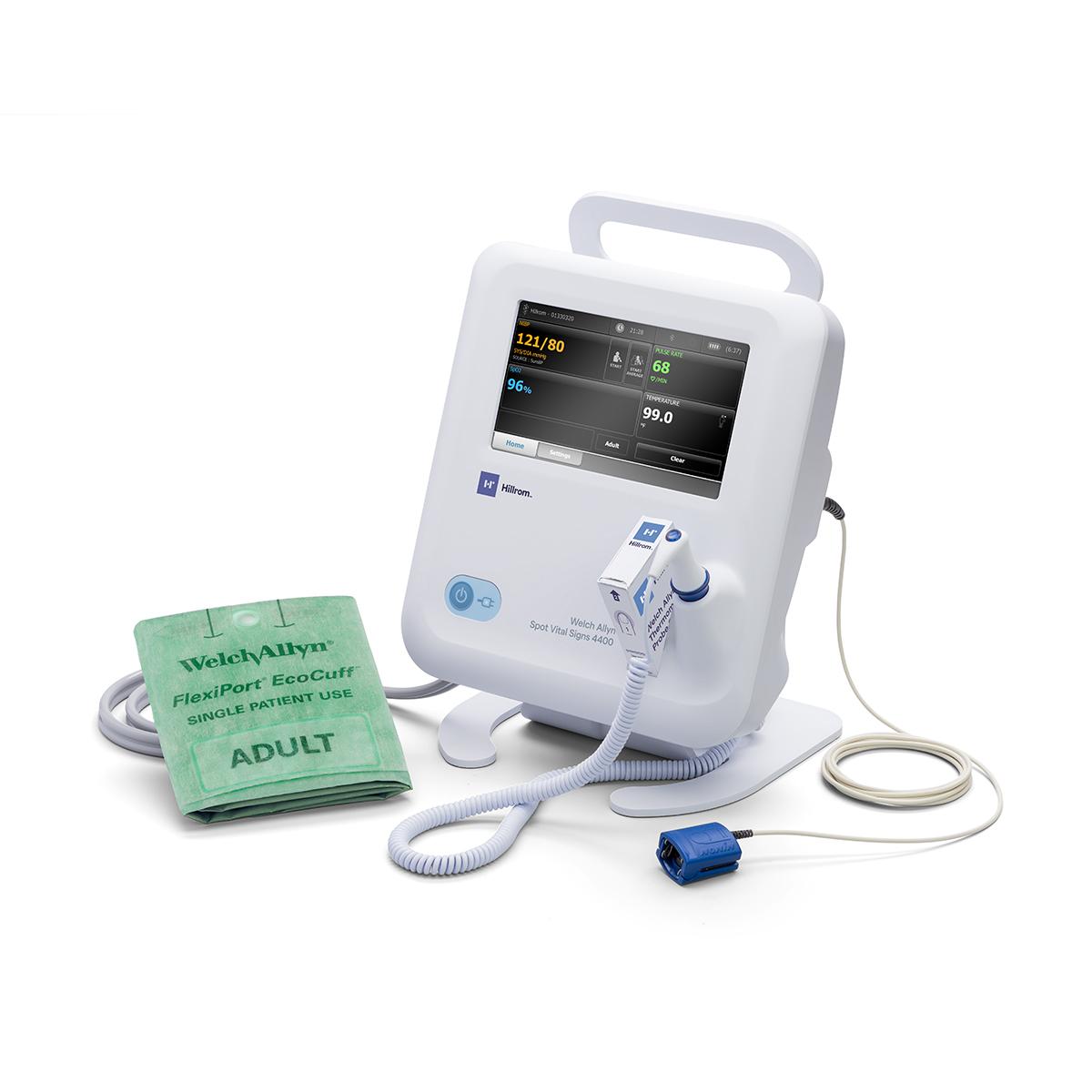
Through our Extended Care Solution, the Welch Allyn® Spot® 4400 Vital Signs Extended Care Device enables patients to take their vital signs at home. Then, with the push of a button, patients can send their readings to a secure, web-based clinical portal for your review and assessment. Consider it a house call without the close quarters.
Welch Allyn® Spot® 4400 Vital Signs Extended Care Device
Take Your Temperature
Hillrom™ Connex® Clinical Portal
Hillrom™ Connex® Patient App
Measure SpO2 & Pulse Rate
Capture a BP Reading
Education & Documentation
Get in the know to get the most value out of your solution.
Product Documentation
-
Brochures
keyboard_arrow_downHillrom™ Extended Care Solution Brochure
-
Installation Guide
keyboard_arrow_downWelch Allyn® Spot Vital Signs® 4400 Assembly Instructions
-
Service Manual
keyboard_arrow_downSpot 4400, Service Manual
Frequently Asked Questions
Expand All-
What cleaning agents can I use on the screen of my Welch Allyn® Spot® 4400 Vital Signs Extended Care Device?
keyboard_arrow_downYes, you can use wipes on the touchscreen surface of the Welch Allyn® Spot Vital Signs® 4400 device as long as the bleach concentration does not exceed 10%.
Note: When cleaning the device, avoid using cloths or solutions that include quaternary ammonium compounds (ammonium chlorides) or glutaraldehyde-based disinfectants.
The following agents are compatible with the device screen. Follow the cleaning agent manufacturer’s guidelines:
Accel INTERVention™
Accel® TB
CaviWipes®
Cleancide®
Clinell® Universal Wipes
Clorox® HealthCare Bleach Germicidal Cleaner
Oxivir® TB
Sani-Cloth® Plus
Sani-Cloth® Bleach
Super HDQ® L10, Dilution rate of ½ oz per gallon of water (1:256) applied to a clean cloth
Super Sani-Cloth®
Tuffie5 Cleaning Wipes
Virex® II (256), Dilution rate of ½ oz per gallon of water (1:256) applied to a clean cloth
10 percent bleach solution, .5% - 1% sodium hypochlorite, applied to a clean cloth
70 percent isopropyl alcohol solution
Accel, Accel INTERvention, Oxivir, and Virex are all trademarks of Diversey, Inc. CaviWipes is a trademark of Metrex Research LLC. Cleancide is a trademark of Wexford Labs. Clinell is a trademark of GAMA Healthcare LTd. Clorox is a trademark of Clorox Co. Sani-Cloth is a trademark of Professional Disposables International, Inc. HDQ is a trademark of Spartan Chemical Co.
-
Where can I obtain extra blood pressure cuffs for my device?
keyboard_arrow_downThe Welch Allyn® Spot Vital Signs® 4400 device utilizes the FlexiPort® EcoCuff® system. If you need to purchase additional cuffs, please visit our online store.
-
Data does not flow from the Welch Allyn® Spot® 4400 Vital Signs Extended Care Device to the Hillrom™ Connex® App
keyboard_arrow_downMake sure app is launched and open to ensure Bluetooth is turned on for your smartphone.
-
The Welch Allyn® Spot® 4400 Vital Signs Extended Care Device can’t read an SpO2, what do I do?
keyboard_arrow_downReposition the clip on your finger, making sure it is lined up with the finger picture on the sensor. Use index finger of hand opposite to the one you are taking a Blood Pressure on. Make sure you don’t have fingernail polish on.
-
The Welch Allyn® Spot® 4400 Vital Signs Extended Care Device screen is dark, what do I do?
keyboard_arrow_downCharge or plug in the device.
-
How do I know my data is going to my physician?
keyboard_arrow_downGo to the app: Settings: Account: look at “Last Sync:” time and date. This will show the last time data was sent to your provider. If that is old, make sure WiFi and Bluetooth are ON in the phone.
-
I have run out of probe covers or BP cuffs, how can I get more?
keyboard_arrow_downContact the facility that you received the device from.
-
I am not sure if my readings were saved.
keyboard_arrow_downOn the app, select one of the options under “Previous Vitals”; check the date and time of the readings
-
My BP cuff gets too painful, what do I do?
keyboard_arrow_downAlways stop a BP if the pressure is too much: Undo the cuff Velcro and reapply as instructed and consult guide in the app (Settings : Help : Take a blood pressure)
-
My Bluetooth will not connect, what do I do?
keyboard_arrow_downRestart the Spot Vital Signs 4400 device and turn Bluetooth off and on again on phone.
Still won’t connect? Unplug the Bluetooth dongle and plug it back in. Then 'forget' the 4400 on your phone. Next, try to repair by taking a set of vitals again.
-
Does this solution fall under the new FCC funding guidelines?
keyboard_arrow_downYes, Not-for-Profit Hospitals, Teaching Hospitals, and Community Health Centers may be eligible to receive federal funding (FCC COVID-19 Telehealth Program Funding) for this type of equipment.
-
What are the reimbursement codes for remote patient monitoring?
keyboard_arrow_downThe CPT codes that can be utilized with this system are: 99453, 99454, 99457.